- Volumes 96-107 (2025)
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• In-situ crystallization of APIs within the carrier achieves process integration.
• The process obviates requirement for high-energy equipment.
• Particle dispersion is achieved through droplets generated by LLPS.
• The ND@SLMs feature high drug loading,low toxicity,and uniform dispersion.
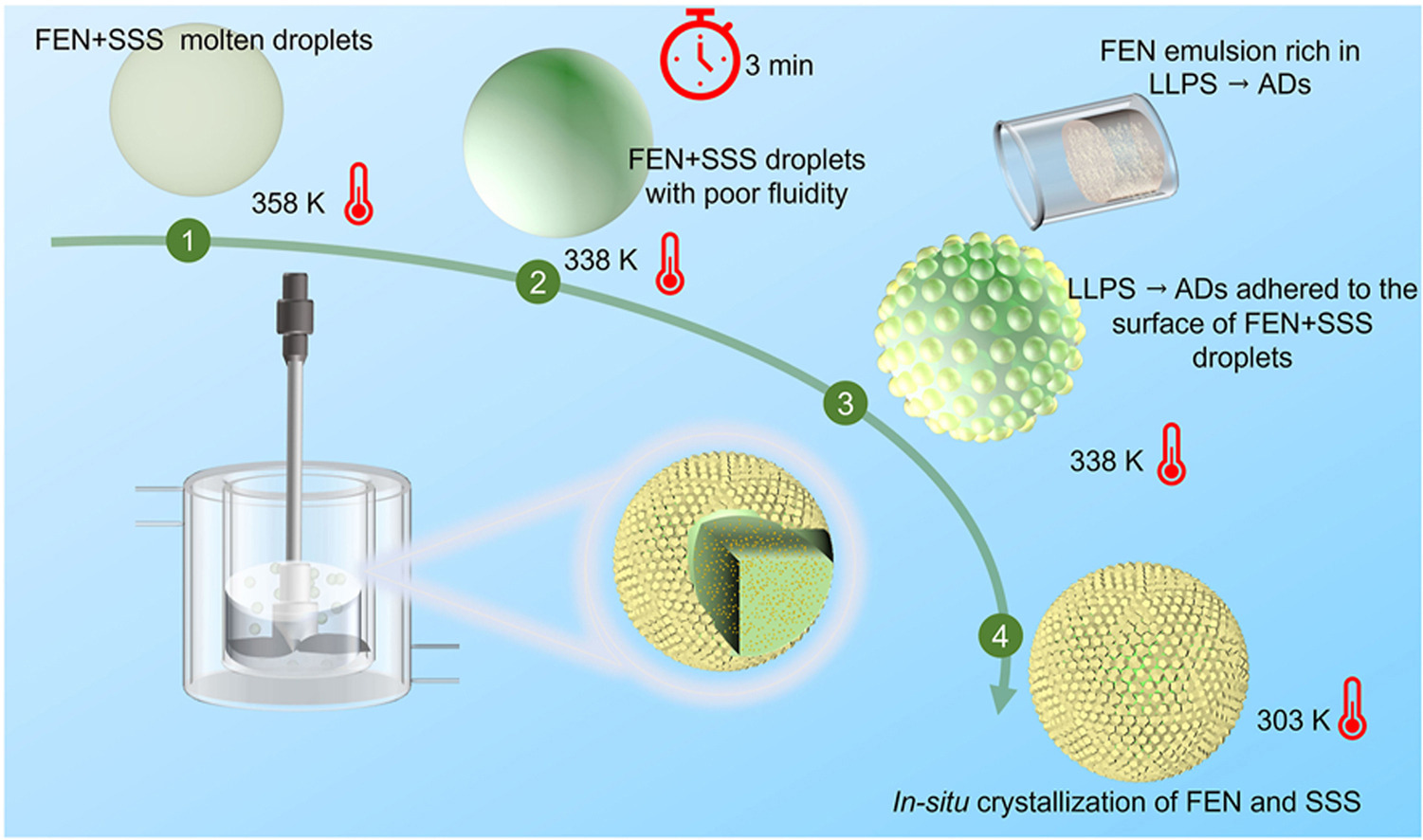
In this work,API nanoscale drugs loaded onto solid lipid microspheres (ND@SLMs) were successfully prepared by combining in-situ crystallization technology and liquid-liquid phase separation (LLPS). This method is characterized by its low energy consumption and the absence of a requirement for high concentrations of surfactants. Tristearin (SSS) was used as the drug carriers,and fenofibrate (FEN) was used as API to verify the feasibility of this method. Characterization was performed using SEM,PXRD, and DSC, while in-situ Raman and EasyViewer enabled real-time monitoring of the particle formation process. The results show that the obtained Active Pharmaceutical Ingredient (API) nanoscale crystals exhibited uniform distribution in the solid lipid carrier and enhanced release rates compared to the bulk ingredients. API droplets prepared by LLPS adhered to the surface of the FEN + SSS droplets played the role of dispersant. Response surface analysis was employed to analyze the independent variables and their interactions, and the optimum value of the processing parameters was obtained. Finally,the expandability of this method to other hydrophobic drugs was verified by ibuprofen (IBU).