- Volumes 96-107 (2025)
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
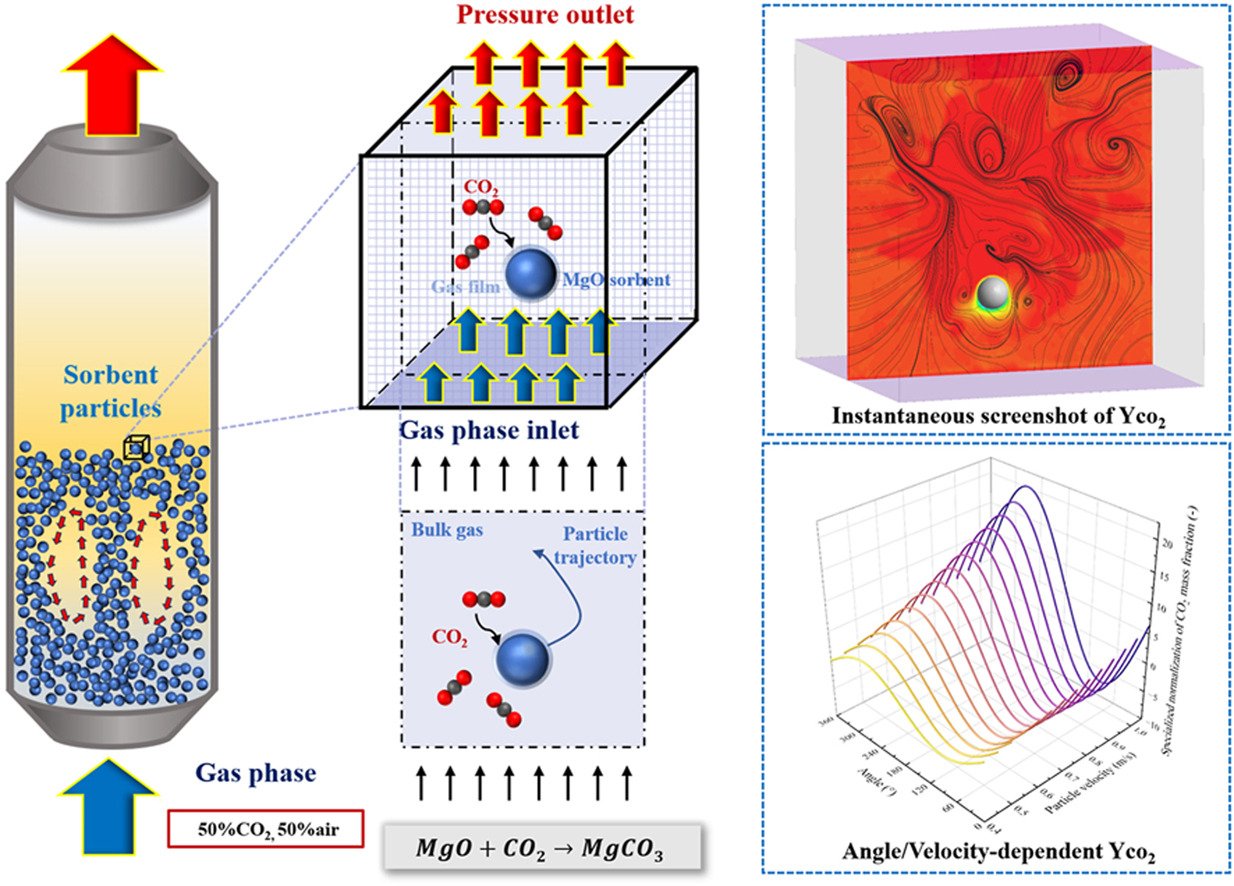
•Numerical model of a single particle was developed.
•Decarbonization performance of a single particle was investigated.
•Effects of particle size,gas velocity,and CO2 mass fraction were studied.
•Correction formula for surface CO2 concentration was proposed.
To obtain a deeper understanding of the mass transfer between MgO-based sorbent particles and gaseous reactants (CO2), it is essential to investigate the mass transfer characteristics of a single moving sorbent particle since most previous researches focused on the removal efficiency of the whole flow field and ignored the behavior of individual particles. Currently, most studies assumed that the reactant (CO2) concentration across the particle surface is uniform based on the average concentration within the grid, while the reactant concentration on the particle surface changes as the particle moves. In this study, the gas-solid mass transfer between CO2 and a moving MgO-based particle was investigated. The results indicated that the motion state and velocity of the particles significantly impact the CO2 removal dynamics and noticeable differences in the CO2 concentration gradient could be observed around the particle. Increasing the reaction temperature, enhancing the CO2 mass fraction at the inlet, appropriately increasing the gas velocity, and selecting an appropriate particle size can significantly enhance the reaction rate,thereby improving the CO2 removal efficiency. The correction formula for surface CO2 concentration and mass transfer reaction rate was also proposed based on the particle's velocity and direction of movement. Based on the correction formula,more detailed guidance for optimizing reactor design could be obtained and the findings provide theoretical guide for designing CO2 removal reactors.