- Volumes 96-107 (2025)
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
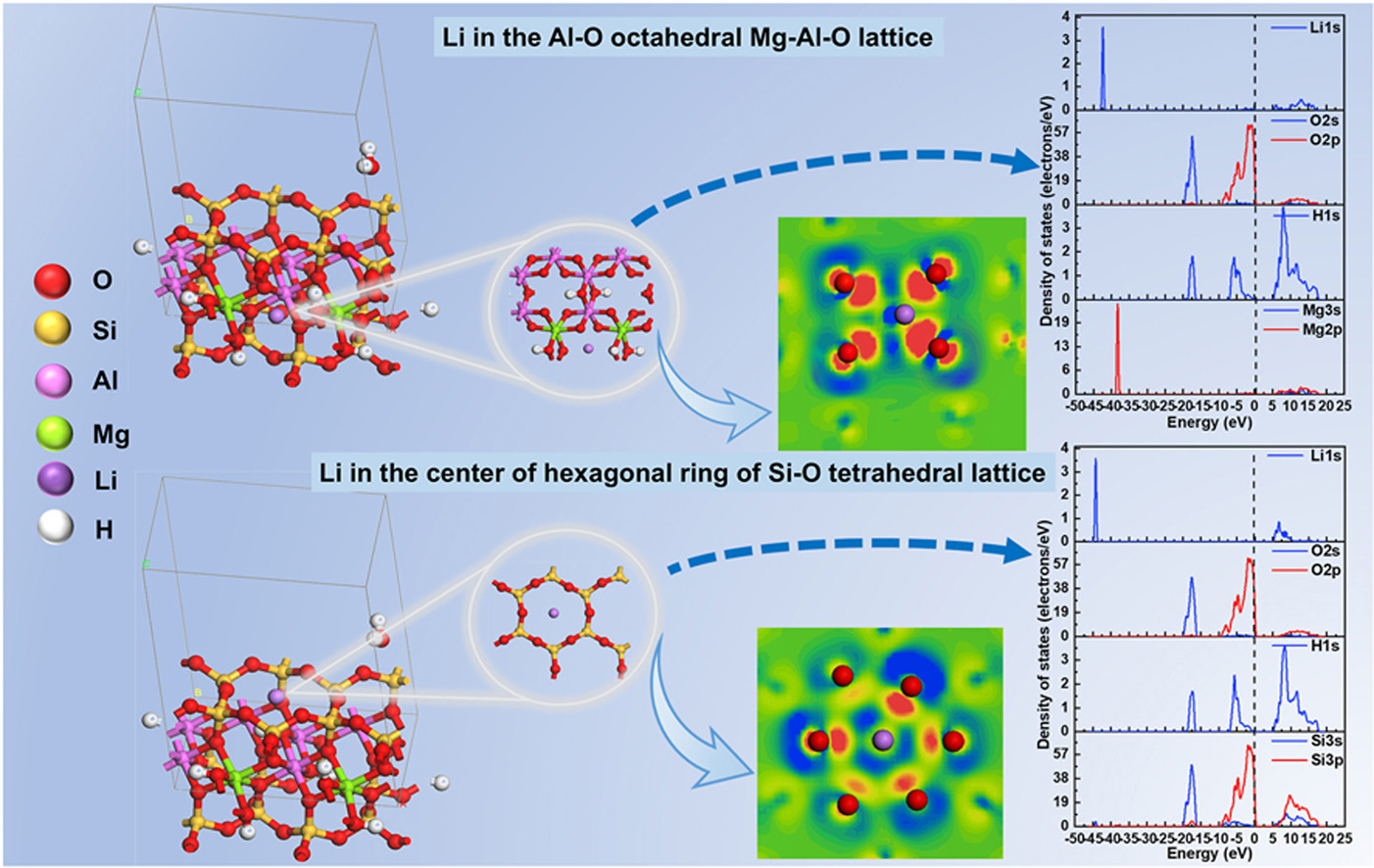
• Dual structural-charge locking mechanism of lithium in octahedral is elucidated.
• Various occurrence sites of lithium in montmorillonite are compared by density functional theory.
• Effects of lithium occurrence sites in montmorillonite on interfacial properties are expounded.
The structural complexity of lithium-bearing clay minerals and limitations of conventional characterization methods impede efficient lithium extraction from montmorillonite-type ores. This study employs density functional theory to elucidate structure-activity relationships governing lithium occurrence in montmorillonite, with particular emphasis on octahedral locking mechanisms and interfacial reaction barriers. Systematic calculations reveal four potential lithium occurrence sites: Al-O octahedra, Si-O tetrahedral lattices, interlayer sites and Li substituted H site. Lithium demonstrates optimal stability within Mg-Al-O octahedral lattices, exhibiting the lowest interaction energy (−672.982 kJ/mol) and substantial Mulliken charge transfer (2.35 e), confirming this configuration as the primary hosting environment. Density of states analysis uncovers critical electronic structure features: the 1s orbital of lithium remains energetically isolated from the Fermi level, explaining its chemical inertness and resistance to direct leaching. Conversely, the reactive 2p orbital of oxygen near the Fermi level facilitate surface interactions with flotation reagents. These electronic signatures imply the feasibility of flotation recovery alongside hydrometallurgical approaches. The octahedral locking mechanism originates from Li-induced dynamic symmetry reconstruction. This process achieves energy minimization through bond-angle regularization, while the notable contraction of Al-O/Mg-O bonds enhances electrostatic coupling. These synergistic effects ultimately establish a structural-charge dual-locking mechanism. This study delivers atomic-level insights into lithium occurrence mechanisms, addressing critical gaps in clay-type lithium mineralogy and revealing structure-activity relationships that guide sustainable lithium recovery via interface regulation.