- Volumes 96-107 (2025)
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
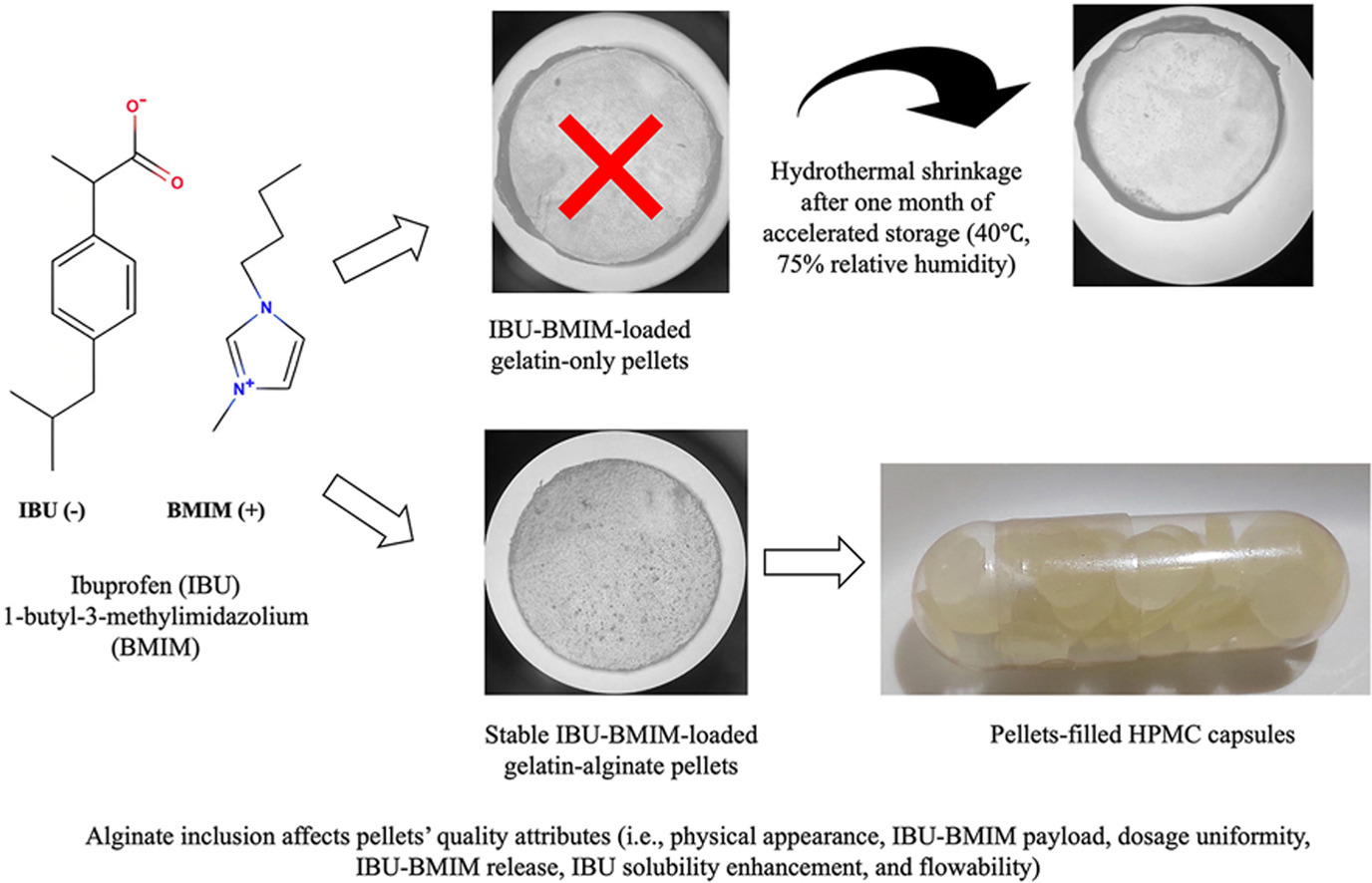
• Alginate affects pellets' quality attributes and gelatin secondary structures.
• Alginate inclusion improves pellets' flowability, slow IBU-BMIM release.
• IBU-BMIM maintains its liquid-like amorphous form in gelatin-alginate pellets.
• IBU-BMIM in pellets preserves its IBU solubility enhancement capability.
• Alginate inclusion stabilizes IBU-BMIM-loaded pellets during accelerated storage.
Ionic liquid (IL) salts of active pharmaceutical ingredients (API) represent promising formulations for poorly-soluble APIs as they eliminate polymorphism commonly associated with conventional API salts. Being highly viscous liquid, oral dosage formulation of API-ILs is challenging, necessitating immobilization onto particulate carriers, typically by spray-drying. This study developed an alternative oral dosage formulation of API-ILs by incorporating them into soft gelatin-alginate pellets, where the alginate's role was to improve storage stability of hygroscopic and thermally-sensitive gelatin. Poorly-soluble ibuprofen (IBU) and 1-butyl-3-methylimidazolium (BMIM) was used as the model API-IL. The impacts of alginate inclusion at varying gelatin-to-alginate ratios on quality attributes of IBU-BMIM-loaded pellets were investigated. The evaluated attributes included physical appearance, IBU-BMIM payload, dosage uniformity, flowability, IBU-BMIM release, and IBU solubility enhancement. The results showed IBU-BMIM remained in its liquid-like amorphous form upon incorporation into the pellets, thereby preserving its solubility enhancement capability, albeit at a lower degree due to slower IBU-BMIM release upon alginate inclusion. Alginate inclusion also influenced the pellets' physical appearance, thickness, flowability, and gelatin's secondary structures, while having minimal impacts on payload and dosage uniformity. Importantly, the pellets remained stable after one-month of accelerated storage (40 °C and 75 % relative humidity) with minimal variations in their quality attributes.