- Volumes 96-107 (2025)
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
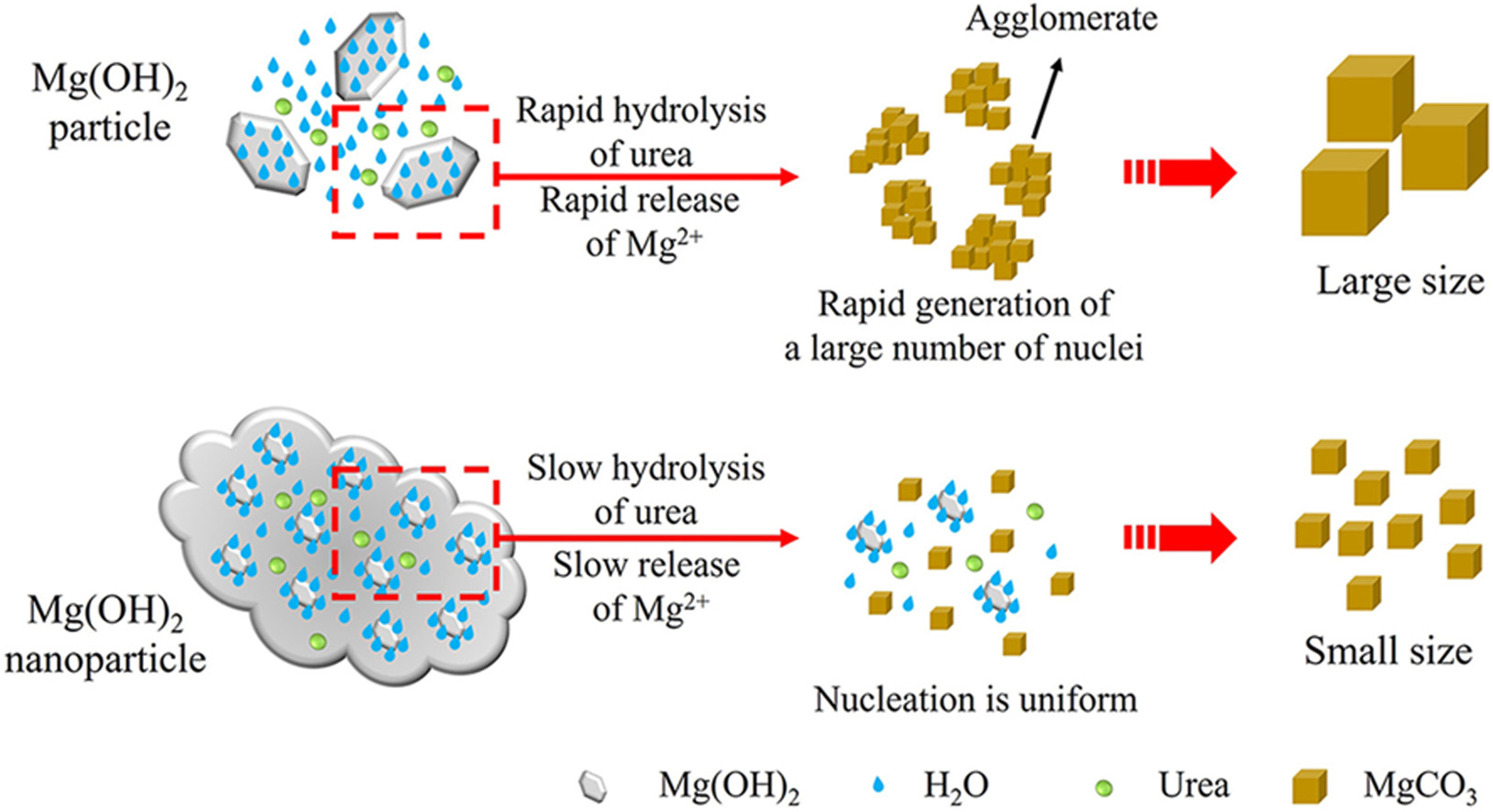
• Uniform small-sized MgCO3 is controllably prepare.
• Presence of Mg(OH)2 colloids inhibits the hydrolysis of urea.
• Uniform small-sized MgCO3 is synthesized from Mg2+ concentrated seawater brine.
• Uniform small-sized MgCO3 has good flame retardant performance.
• High purity MgO with a purity of 99.54% was prepared by using MgCO3 as precursor.
The large and uneven grain size of anhydrous magnesium carbonate (MgCO3) seriously restricts its application ranges and performances. In this study, we proposed a controllable and cost-effective strategy to synthesize uniform small-sized MgCO3 from Mg2+ concentrated seawater brine in the absence of crystal modifiers. In this process, solid NaOH was directly added to Mg2+ concentrated seawater brine to completely and rapidly convert Mg2+ to magnesium hydroxide (Mg(OH)2) nanoparticles. These nanoparticles are redispersed in water to form the colloidal system, where Mg(OH)2 nanoparticles hydrothermally reacts with urea to obtain uniform small-sized MgCO3 particles. The influence of reaction temperature, reaction time, and the molar ratio of magnesium ions to urea on the synthesis of MgCO3 is systematically investigated. In the highly-dispersed and stable colloidal system, Mg(OH)2 nanoparticles could exert an effective and sustained retarding effect on the hydrolysis rate of urea by attracting free water, resulting in the controllable release of NH4+, CO32−, and Mg2+. This study presents a simple route to realize the controllable synthesis of uniform small-sized MgCO3 particles, and demonstrates the feasibility of using MgCO3 as an ideal filler for enhancing the performance of polymers as well as an ideal precursor for high-purity MgO production.